Nanoparticles for Disease Diagnostics and Drug Delivery
We design, patent and publish nanoparticles for disease diagnostics based on Raman scattering. Recent work on therapeutics has focused on lipid nanoparticles (LNPs), which we currently use for transfection in vitro and in vivo. Particular focus recently has been LNPs to treat leukemia and lung disease/injury. While the in vitro work is typically in our labs, the in vivo work is done in collaboration with clinicians at Mt Sinai and St Michael’s hospitals.
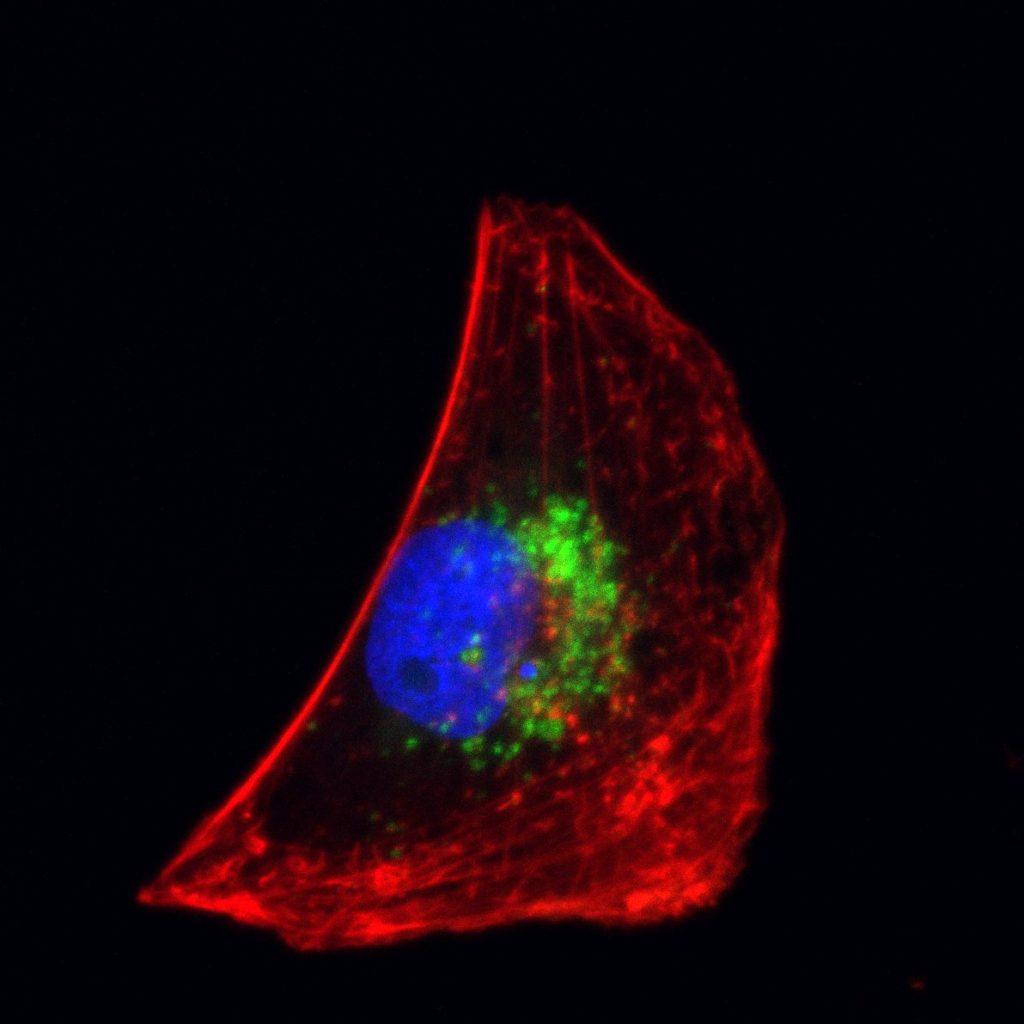
LNPs (green) in a BEAS-2B cell, from a normal bronchial epithelial cell line, image by Amin Ektasabi from Claudia dos Santos’ lab.
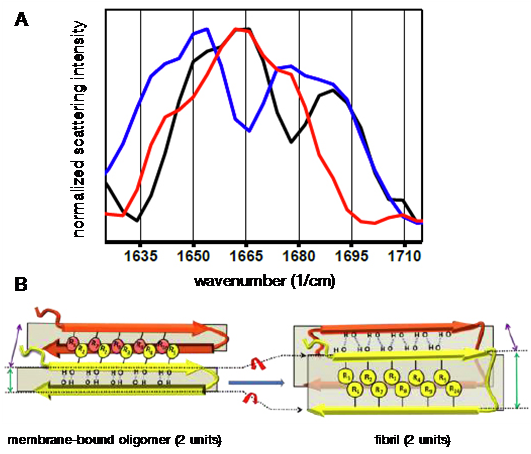
(A) Surface-enhanced Raman scattering spectra of lipid membrane-bound amyloid-beta (Aβ): unlabelled Aβ40 (black), 13C- and 15N-labelled Aβ40 at D23 and K28 (red), and 13C- and 15N-labelled Aβ40 at E11, F19, A30, L34, V36, and G38 (blue). (B) Aβ backbone reorientation revealed by the spectra, which may be compared to the fibril form. From Bhowmik, D. et al. Cell-Membrane-Mimicking Lipid-Coated Nanoparticles Confer Raman Enhancement to Membrane Proteins and Reveal Membrane-Attached Amyloid-β conformation. ACS Nano 2015, 9, 9070–9077. doi: 10.1021/acsnano.5b03175.
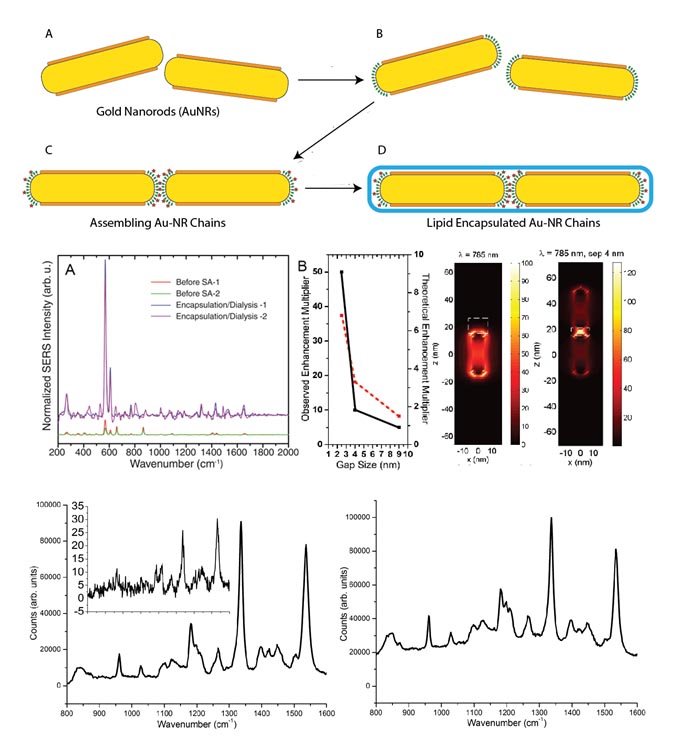
Gold nanorods may be assembled using polystyrene linkers, achieving plasmonic hot spots in the gaps between the nanorods. Surface-enhanced Raman scattering may be enhanced by at least 750X. Even shorter gaps can be achieved with porphyrins/phthalocyanines, leading to enhancement factors of at least 3500. Lipid encapsulation results in stable species for weeks to months and allows for eventual functionalization with antibodies for biological use.
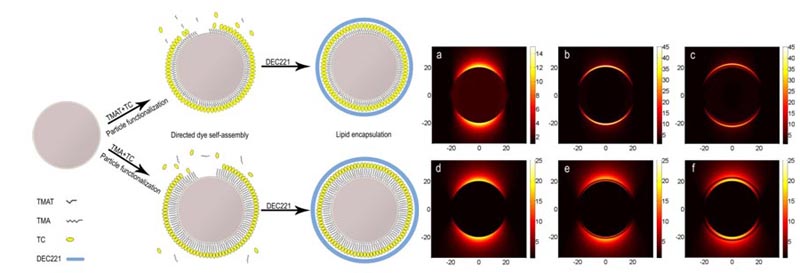
(a) Naked particle; (b–c) With linker layers respectively coated in TC J-aggregate. Irradiated at co-resonant wavelength the electric field is strongly confined within the J-aggregate layer; (d–f) Irradiated at off-resonant wavelength. The electric field is measured in (V/m)2, and the distance from the center of the particle in nm.
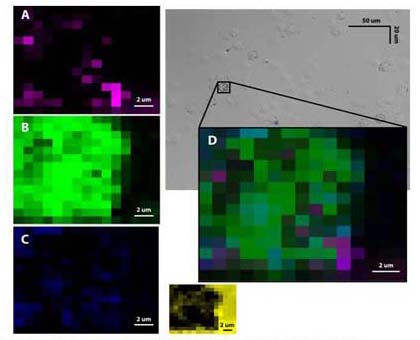
3-plexed labeling of LY10 B cell lymphoma line using SERS Au NPs targeted to CD45, CD20 and CD5, respectively. By fitting each experimental spectrum from cells to SERS spectra of each nanoparticle label using least-squares fitting, regions labelled by each probe can be determined. Regions of best fit to each probe type: (A) Anti-CD45 SERS NPs, (B) anti-CD20 SERS NPs, (C) anti-CD5 SERS NPs, (D) overlay of 3 Raman maps. Inset: regions of best fit to sample background.
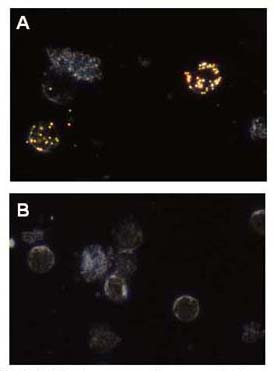
Dark field microscopy images of primary chronic lymphocytic leukemia cells labelled with anti-CD20 SERS NPs. 60 nm spherical NPs scatter green-gold in the images, background cell scattering is blue-white. (A) Anti-CD20 SERS NPs, (B) non-specific IgG1-SERS NP controls.

Jurkat 48 hr after GFP transfection. Left, fluorescence image. Right, Flow cytometry showing cells ~73% positive for GFP.
Representative publications
Ip, S.; MacLaughlin, C. M.; Joseph, M.; Mullaithilaga, N.; Yang, G.; Wang, C.; Walker, G. C. Dual-Mode Dark Field and Surface-Enhanced Raman Scattering Liposomes for Lymphoma and Leukemia Cell Imaging. Langmuir 2018. Article ASAP. doi: 10.1021/acs.langmuir.8b02313.
Bhowmik, D.; Mote, K. R.; MacLaughlin, C. M.; Biswas, N.; Chandra, B.; Basu, J. K.; Walker, G. C.; Madhu, P. K.; Maiti, S. Cell-Membrane-Mimicking Lipid-Coated Nanoparticles Confer Raman Enhancement to Membrane Proteins and Reveal Membrane-Attached Amyloid-β Conformation. ACS Nano 2015, 9, 9070–9077. doi: 10.1021/acsnano.5b03175.
Xu, X. G.; Ghamsari, B. G.; Jiang, J.-H.; Gilburd, L.; Andreev, G. O.; Zhi, C.; Bando, Y.; Golberg, D.; Berini, P.; Walker, G. C. One-dimensional surface phonon polaritons in boron nitride nanotubes. Nat. Commun. 2014, 5, 4782. doi: 10.1038/ncomms5782.
